The Pfizer Covid-19 vaccine medicine name is actually ‘BNT162b2 [mRNA]’. It is called that because it was produced by German company BioNTech but is actually called COMIRNATY™ which is the trading name of the vaccine. The active ingredient in Comirnaty is ‘tozinameran‘ (0.5mg). However, this too is a trade name, established in two parts, tozina-, is the invented prefix required by the World Health Organization, while the second half -meran is the required suffix for new mRNA vaccines.
You have probably heard of the term mRNA, this is basically Messenger Ribonucleic Acid (mRNA) – which is the Active Ingredient of BNT162b2. It is a modified Nucleoside which is a ‘Chemical messenger (Gene Therapy*), encoding the viral spike glycoprotein (S) of SARS-CoV-2, to instruct cells to make proteins that mimic the outer surface of the new coronavirus. mRNA is highly unstable and therefore has to be encapsulated in Lipid Nanoparticles (see below). Basically mRNA is a synthetic mimicry of a protein that stimulates cells to produce a spike protein with the same sequencing as a covid-19 spike.
In fact, the actual coding sequence (CDS) of the Covid-19 mRNA is secret – due to the CDC (USA) and NIH (USA) owning the patent of the sequence and not releasing it publicly. You can see in the diagram below, the make up of the mRNA, includes two further untranslated regions (UTRs). This means the ‘ingredients’ of the active component of the vaccine are unknown or untranslated.
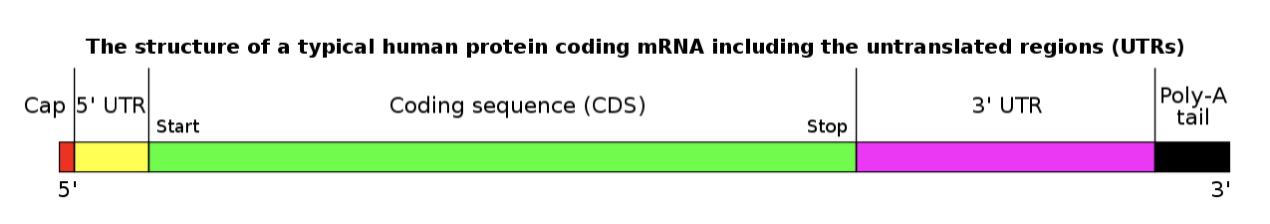
No prior mRNA vaccine has been approved before 2021 and therefore there is no safety data available. But a closer look at the known ingredients surrounding the mRNA tells a different story.
*According to the Encyclopaedia Britannica – mRNA changes the structure of the DNA.
SO WHAT’S IN IT?
In order to get mRNA from injection into the cells of the body, it needs a molecular escort. By itself, mRNA cannot freely cross our cell membranes. Lipid nanoparticle technology solves this problem by packaging mRNA — known as a transcript — into a complex vesicle of phospholipid molecules that are designed to fuse with our body’s own cell membranes. The lipid nanoparticle wall of the BNT162b2 vaccine is made up of four different compounds, some of which are known corrosive irritants, allergens, and restricted for use in food, drug or households:
- ((4-hydroxybutyl)azanediyl)bis(hexane-6,1-diyl)bis(2- hexyldecanoate) a.k.a. ACL-0315 — A proprietary phospholipid that makes up the basic structure of the nanoparticle wall. These molecules are ‘modelled’ after native phospholipids found in living cells.
- 2-[(polyethylene glycol)-2000]-N,N-ditetradecylacetamide a.k.a. ACL-0159 — Another commercially available phospholipid linked to polyethylene glycol (a component of anti-freeze).
- 1,2-distearoyl-sn-glycero-3-phosphocholine — A commercially available glycophospholipid used as a component of the cell wall.
- Cholesterol — A native molecule ubiquitous in the human body. Cholesterol aids in the fluidity or rigidity of a lipid membrane depending on temperature.
Beyond the compounds used in making the nanoparticle, there are some ingredients used to control the aqueous [water dissolving] environment that the vaccine is delivered in. These ingredients enhance the stability of the nanoparticle, and control against swings in pH. These include:
- Potassium chloride — Simple salt of potassium (K+) and Chloride (Cl-), two ubiquitous elements found in the body. However the dosages are unknown and the LabChem Safety Data Sheet restricts use from food, drug or household.
- Dipotassium phosphate — A potassium salt of dihydrogen phosphate, a naturally occurring compound. Used as a buffer to protect against swings in pH. Listed as a possible irritant.
- Sodium chloride — Laboratory-grade table salt. However this is a metal halide, that when injected can cause excessive blood loss, fever.
- Disodium phosphate — Similar to dipotassium phosphate, this is a sodium salt of dihydrogen phosphate. Also used as a buffer. Listed as a corrosive irritant.
- Sucrose — Laboratory-grade table sugar. Used to stabilize nanoparticles during transport.
Because these vaccines need to be stored at very cold temperatures, there is no need for preservative agents. Note that the Australian TGA Product Information (PI) document list the vaccine as being potassium and sodium free due to the low dosages.
WELL DID YOU KNOW…
The Australian TGA Product Information (PI) (version: pfpcovii11021) also states the methods and conditions the vaccine is to be stored, transported and administered. An excerpt of the Shelf Life chapter appears below:
6.3 Shelf life
In Australia, information on the shelf life can be found on the public summary of the Australian Register of Therapeutic Goods (ARTG). The expiry date can be found on the packaging.
Unopened vial
9 months at -90°C to -60°C.
Unopened vials may be stored and transported at -25°C to -15°C for a total of 2 weeks and can be returned to -90ºC to -60°C.
Once removed from the freezer, the unopened vial can be stored for up to 1 month at 2°C to 8°C. Within the 1 month shelf-life at 2°C to 8°C, up to 12 hours may be used for transportation.
Prior to use, the unopened vial can be stored for up to 2 hours at temperatures up to 30°C.
Once thawed, COMIRNATY should not be re-frozen.
Diluted medicinal product
Chemical and physical in-use stability, including during transportation, has been demonstrated for 6 hours at 2ºC to 30ºC after dilution in sodium chloride 9 mg/mL (0.9%) solution for injection. From a microbiological point of view, the product should be used immediately. If not used immediately, in-use storage times and conditions are the responsibility of the user.
6.4 Special precautions for storage
Store in a freezer at -90°C to -60°C.
Store in the original package in order to protect from light.
During storage, minimise exposure to room light, and avoid exposure to direct sunlight and ultraviolet light.
Thawed vials can be handled in room light conditions.
When you are ready to thaw or use COMIRNATY:
Transfers of frozen vials stored at ultra-low temperature (<-60°C)
• Closed-lid vial trays containing 195 vials removed from ultra-low temperature frozen storage (<-60°C) may be at temperatures up to 25°C for up to 5 minutes for transfer between ultra-low-temperature environments.
• Open-lid vial trays, or vial trays containing less than 195 vials removed from ultra-low temperature frozen storage (<-60°C) may be at temperatures up to 25°C for up to 3 minutes to remove vials or for transfer between ultra-low-temperature environments.
• After vial trays are returned to ultra-low temperature frozen storage following temperature exposure up to 25°C, they must remain in ultra-low temperature frozen storage for at least 2 hours before they can be removed again.
Transfers of frozen vials stored at -25°C to -15°C
• Closed-lid vial trays containing 195 vials removed from frozen storage (-25°C to -15°C) may be at temperatures up to 25C for up to 3 minutes.
• Open-lid vial trays, or vial trays containing less than 195 vials, removed from frozen storage (-25C to -15°C) may be at temperatures up to 25°C for up to 1 minute.
Once a vial is removed from the vial tray, it should be thawed for use.
Transportation
If local redistribution of unopened vials is needed, and full trays containing vials cannot be transported at -90°C to -60°C, available data support physical and chemical stability during transportation of 1 or more thawed vials at 2°C to 8°C for up to 12 hours. Any hours used for transport of unopened vials at 2°C to 8°C count against the 1 month limit for storage at 2°C to 8°C.
If local redistribution of diluted medicinal product in vials or syringes is needed, available data support physical and chemical stability during transportation at 2°C to 30°C for up to 6 hours. Any hours used for transport of diluted medicinal product in vials or syringes at 2°C to 30°C count against the 6-hour limit for storage at 2°C to 30°C. Microbiological risks and package integrity, particularly for prepared dosing syringes, are the responsibility of the preparer during transportation of diluted medicinal product.
One wonders how often these protocols are being followed, and if not followed, what the implications are for people receiving the vaccine. If you, or someone you know, has seen the use of vaccines in a manner other than that described in the TGA Product Information Document, then report it.
WHAT TO DO ABOUT IT…
If you have evidence of malpractice report it to https://www.ahpra.gov.au/Notifications.aspx
If you think using this technology is a breach of law – contact your State MP representative.
If you think someone else needs to know this information – forward it on.
References
https://thegreatawakening.jimdofree.com/pfizer-biontech/
https://www.britannica.com/science/messenger-RNA
https://coronavirus.medium.com/detailing-pfizers-covid-19-vaccine-ingredients-e9cf0519dede
https://gsrs.ncats.nih.gov/app/substance/6fefa717-6a4c-435f-a692-46189283764f
https://www.rxreasoner.com/substances/tozinameran/pharmacology


Leave a Reply